The measurement of pH, always represented by a small “p” and a capital “H,” is a measure of the amount of hydrogen ions (H+) present in a substance such as water. Knowing the amount of hydrogen in a substance allows us to judge whether it is acidic, neutral, or basic.
The pH scale ranges from 0 (most acidic) to 14 (most basic), with the value of 7 representing neutral solutions. As we go down the pH scale, each unit of pH value represents a tenfold increase in how much hydrogen is present. (For example, water with a pH of 7.5 is 10 times more acidic than water with a pH of 8.5.)
Most natural waters in the United States have pH values between 6.5 and 8.5. Rain usually has a pH of between 5.0 and 6.0, but the “acid rain” that we hear about has pH values that average around 4.3.
Lakes that receive a great deal of acid rain may have water with pH values as low as 4. (From this, we can see that low pH values are more acidic than higher values.) The Great Lakes are protected from acid rain by limestone bedrock, which acts as a buffer to neutralize acid rain.
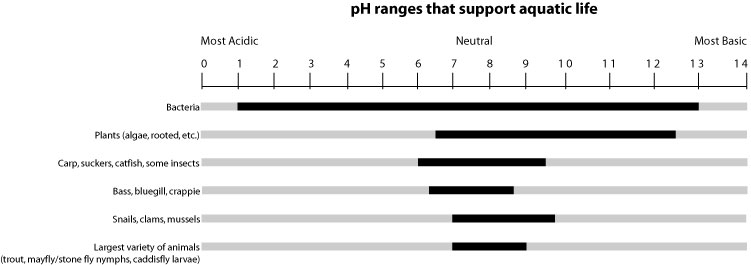
The pH level of the water in rivers, lakes, and wetlands is important to plant and animal life. Most animal species cannot survive if the water is too acidic (generally below 5.0), or too basic (above 9.0). Optimal pH for many species is between 7.0 and 9.0. pH levels can often indicate the types of animals found in a particular habitat. For example, bass and bluegill might be found at pH 8.5, but trout and mayflies will not be found in the same area. On the other hand, bass, bluegill, trout, and mayflies might be found in a habitat with a neutral pH of 7.0.

